Menu
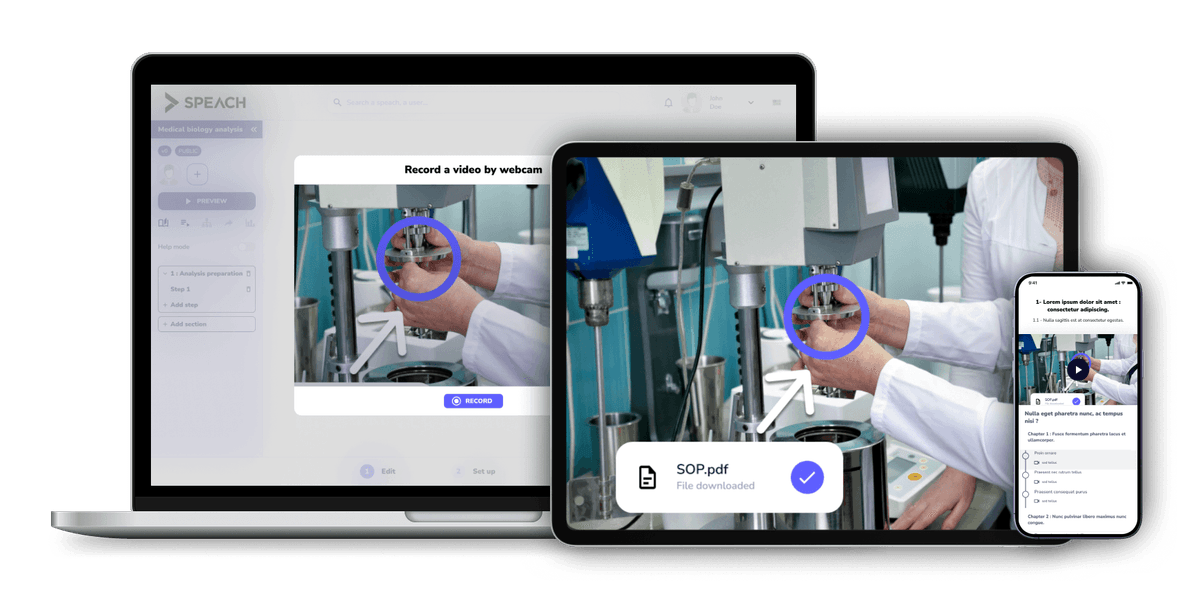
Speach's platform delivers high-quality, GxP-compliant training and work instructions. FDA 21 CFR Part 11 Compliant.
SAML/LDAP authentication - safer integrations across your stack.
Fully compliant with FDA CFR 21 Part 11 and EMA Eudralex Annex 11 (electronic records and signatures)
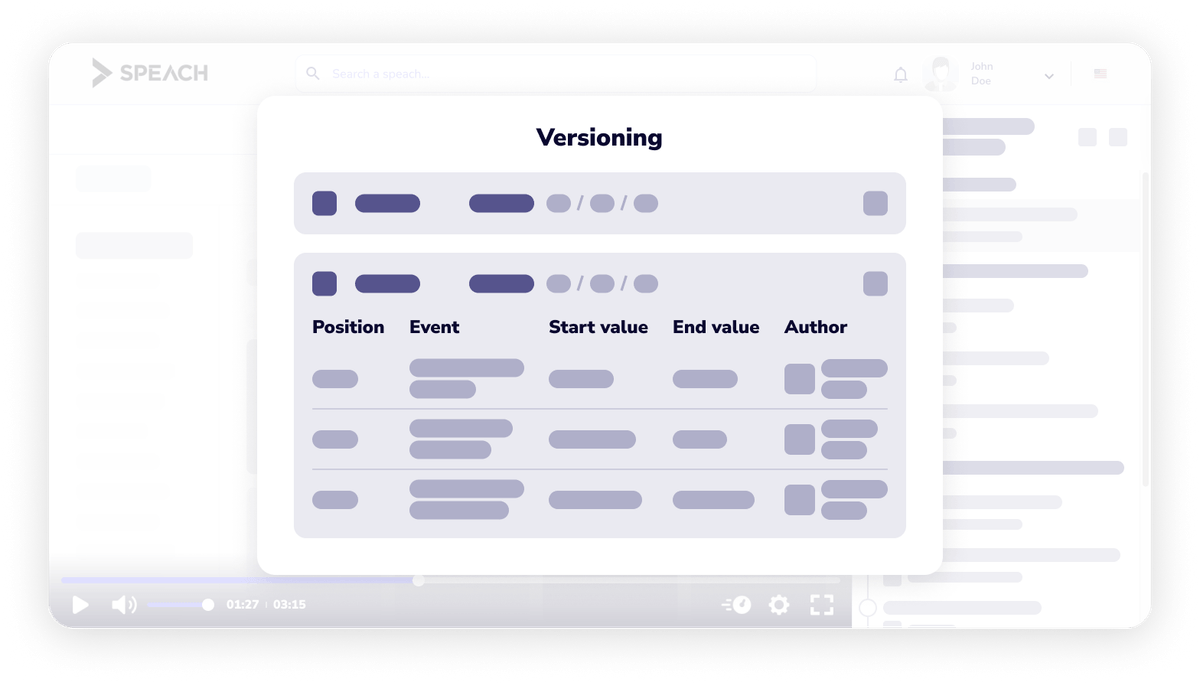
Detailed audit trails and powerful search facilitate better audits and inspections.
Once captured or created, data is never lost.
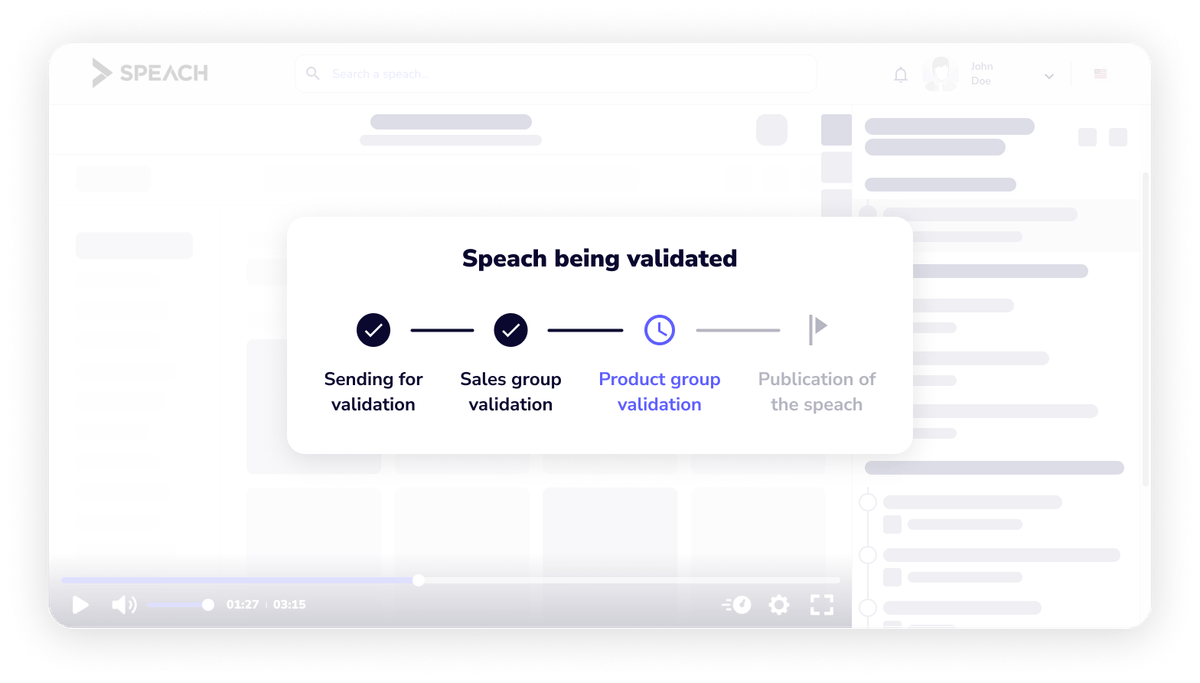
Automated review and validation workflows across the content lifecycle.
Long-term support (LTS) validated version and documentation detailing every product change.
As a Quality Director in the pharmaceutical industry, finding an effective way to deliver GxP-compliant training was challenging until we started using Speach. This microlearning platform transformed our approach to training. Its GxP-compliant features made complex regulations understandable and engaging for our team, and the ability to quickly update content ensures we're always compliant.
The tracking and reporting feature has been a standout, making audit preparations simpler and more efficient. With Speach, we've seen a noticeable improvement in employee engagement and retention of GxP concepts, and the reduced training time has been a significant cost-saver.
Speach is an invaluable tool for any pharmaceutical company seeking efficient, compliant, and effective training solutions.
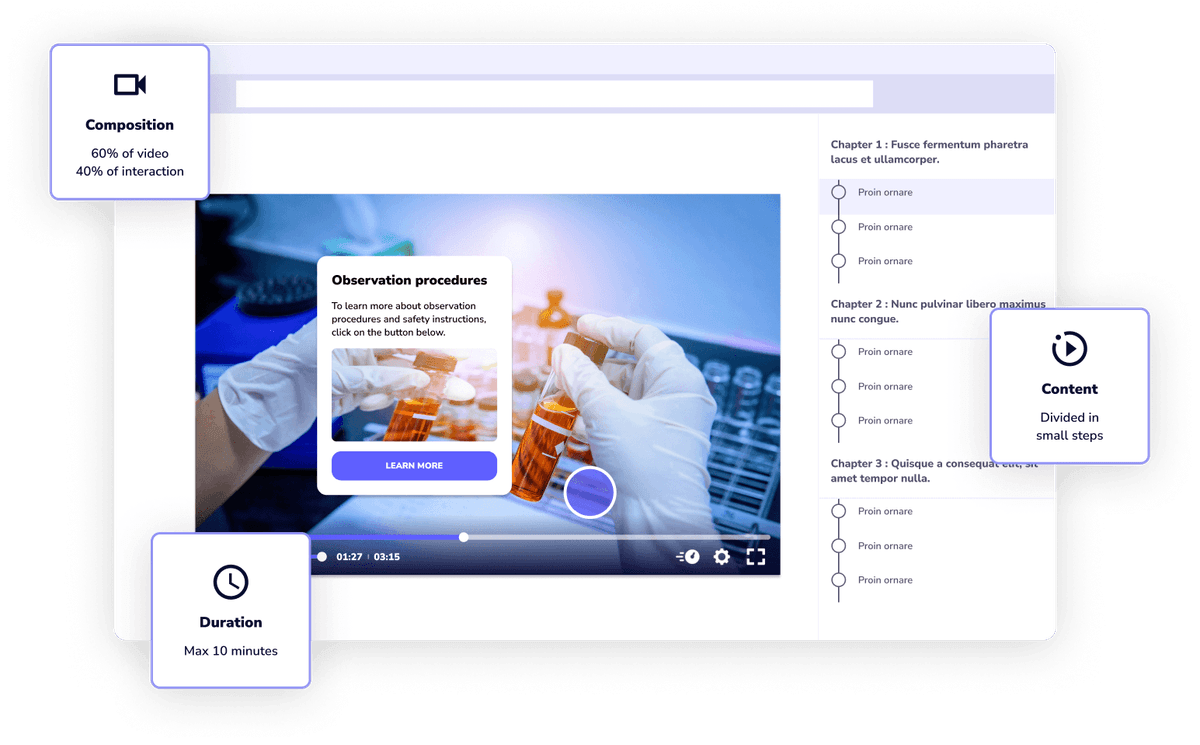
Speach is not just another tool for creating GxP compliant training; it's an entire ecosystem dedicated to effective learning. Beyond its powerful video creation capabilities, Speach integrates micro-learning principles, interactive elements, and real-time collaboration features. This holistic approach ensures not only the production of engaging content but also its effective delivery, fostering deeper understanding and lasting impact.